What services ?
OUR MISSIONS
To explore the medical and industrial potential of iPS cells.
To bring innovative technology solutions by providing expertise in all stages of project development and translation.
-
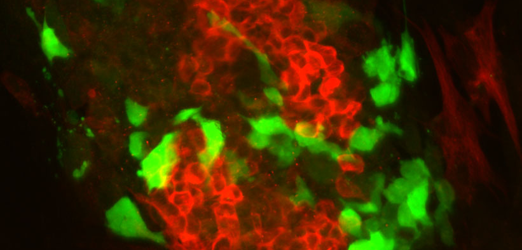 Colony of murine differentiated embryonic stem cell, expressing a reporter of paraxial mesoderm (green), and a neural protein (red). © IGBMC
Colony of murine differentiated embryonic stem cell, expressing a reporter of paraxial mesoderm (green), and a neural protein (red). © IGBMC -

-
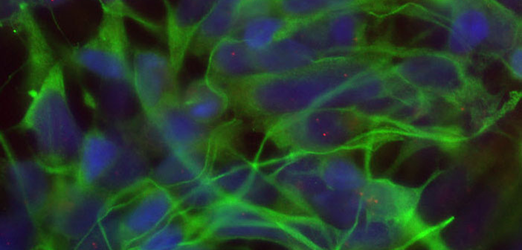
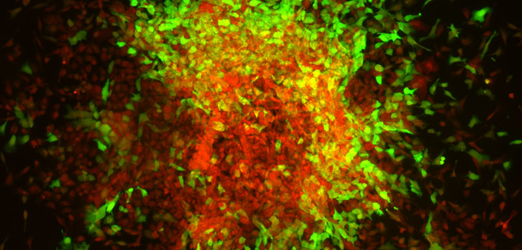 Cells undergoing differentiation with reporter gene
Cells undergoing differentiation with reporter gene -
 Cryogenic storage of cell lines bank © AFMTELETHON / Luc Morvan
Cryogenic storage of cell lines bank © AFMTELETHON / Luc Morvan -
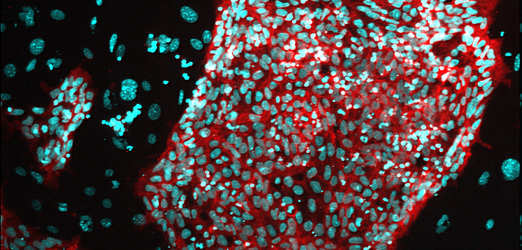 Colony of Macaque ES cells ©PrimaStem
Colony of Macaque ES cells ©PrimaStem -
-
 Cellular high throughput screening platform. Biocel System - Agilent Technologies. © IGBMC
Cellular high throughput screening platform. Biocel System - Agilent Technologies. © IGBMC -
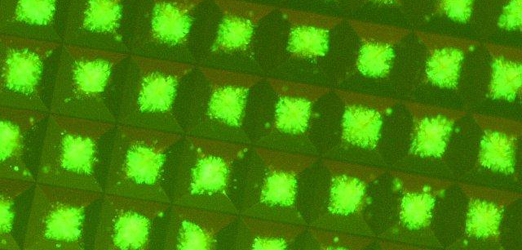 Fluorescent iPS cell colonies in microwell plates Aggrewell StemCell Technologies. © ESTEAM Paris Sud
Fluorescent iPS cell colonies in microwell plates Aggrewell StemCell Technologies. © ESTEAM Paris Sud -
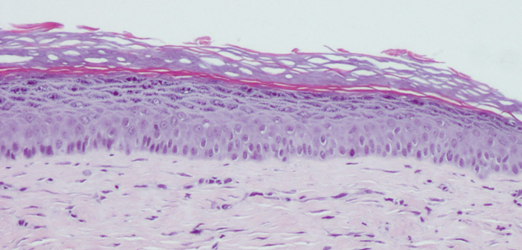 Epithelium formed from human embryonic stem cells. © AFMTELETHON / Luc Morvan
Epithelium formed from human embryonic stem cells. © AFMTELETHON / Luc Morvan -
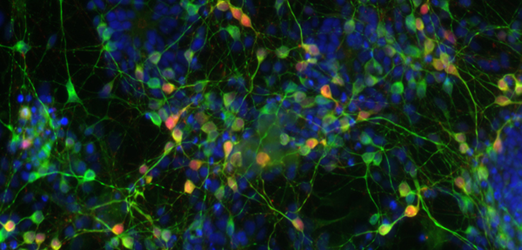
 Aggregates of neurons carrying the mutation DM1 (Myotonic Dystrophy Type I) differentiated from human embryonic stem cells © I-Stem
Aggregates of neurons carrying the mutation DM1 (Myotonic Dystrophy Type I) differentiated from human embryonic stem cells © I-Stem
Collaboration and co-development
By providing support for the overall development of a stem cell project from conception to industrialization, INGESTEM offers a comprehensive set of resources and partnerships on cellular reprogramming strategies, methods of differentiation and tissue engineering, molecular high-throughput screening, and the early stages of clinical trials.
Services
INGESTEM offers a broad range of services, including analysis of unmet needs on the field of stem cells, implementation of technological prototypes and validated tests.
Training
INGESTEM organizes a network of scientific events and international conferences. Training courses on pluripotent stem cells are organized as practical workshops.
OUR GOALS
INGESTEM aims to coordinate and structure different steps involved in the development stem cells used either for drug screening or for protocols of regenerative medicine with the following strategies:
- Creation of a “research” biobank of human and animal induced pluripotent stem cells (iPS) and embryonic stem cells (ES)
- Development of a french registry of iPS cell lines qualified with international standards, including clinical and biological annotations
- Cell and tissue engineering
- Risk analysis and characterization of products from cell engineering
- Standardization of protocols and automated production
- Transgenic animal models
- Modeling of human monogenic and polygenic genetic diseases, drug discovery by high throughput screening.
- Therapeutic models with design and validation of preclinical models to the production of Advanced-Therapy Medicinal Products (ATMPs) for early clinical trials phase I-II.